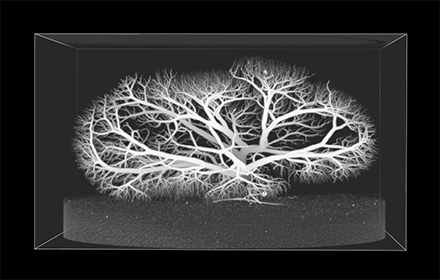
Vascular Extraction & Analysis
Vascular Reconstruction
The rapid advancement of high resolution imaging modalities, from micro-CT to confocal microscopy, has enabled us to capture vast amounts of information from minute subjects. Specimens can be imaged down at a resolution of micrometers generating billions of voxels from a volume no bigger than the size of a golf ball. Whereas in small datasets it is easy to segment the areas of interest manually by hand, as the amount of data increases exponentially with imaging volume, so does the need for automated image processing tools. This is particularly true in the case of vessel imaging, where micro-scale resolution can give rise to millions of vascular segments.
For this reason we have developed a fast, robust and accurate vascular segmentation pipeline. Vascular Extraction Software (VasEx) can reliably reduce high resolution vascular images to a topological representation of the vascular network with subvoxel accuracy on the position and radius of vessel segments. Our pipeline consists of multiple stages including denoising, automated vessel detection and segmentation, skeletonisation and per-node radius estimation. In addition we also include a topology-correction and quality control algorithm to prune out anatomically unrealistic vascular segments that could result from imaging artifacts. VasEx has been successfully tested on images from various modalities and anatomical domains including high resolution cryomicrotome images of porcine hearts, micro-CT systemic, coronary and renal vasculatures and even thoracic CT and MR datasets.
More information on VasEx can be found in the Tools Section.
Vascular Scaling Law
The determinants underlying the formation and structure of a vascular network (the 'design principles') have long been a subject of theoretical investigation. From the pioneering work of Murray (1926) on minimum energy expenditure to numerous hypotheses that followed, vessel dimensions in networks have been mathematically characterised through scaling laws. In the coronary circulation, the detailed anatomical analyses conducted in 90s led to several refinements.
These developments, however, have exclusively assumed a steady-state flow which is a poor approximation for the coronary system, particularly given the recent recognition of the importance of wave dynamics in coronary flow. Our recent research has therefore reconciled the theory underlying pulsatile flow with the framework of scaling laws, resulting in a new model with which to assess the structural determinants. In conjunction with high-resolution reconstruction of coronary vasculature obtained with VasEx, it was shown that the optimal scaling according to the previously developed scaling laws depend on the pulsatility of the flow (quantified by Womersley number), and that there is a critical scale (around 100 microns diameter) below which the governing factors for optimal scaling transitions into a different regime. These observations were fairly consistent across species (pig, dog, human). This work is detailed in our upcoming publication, here.
Myocardial Territorial Analysis
From the high-resolution reconstruction it is also possible to delineate the myocardial boundaries, allowing a reconstruction of the tissue volume surrounding the vascular network. From this, we have conducted a multi-scale analysis of flow territories belonging to every vessel segment in the network, creating a detailed map of regional vessel-myocardial coupling.
To date, this data has been used to assess the skimming effects of microsphere transport in the network (Sinclair et al, 2015), which is a gold standard technique for flow quantification against which other methods (e.g. perfusion MR imaging) are compared. This has identified a previously unreported role of microsphere skimming at large vessel junctions which has strong implications for flow quantification. This finding suggests that when using thin transmural segments to quantify flow from microspheres, a skimming-related deposition bias may result in underestimation of perfusion in the subepicardium, and overestimation in the subendocardium.
Work is currently under way to apply the flow territorial data to examine the transmural and supply vessel-wise (LAD/LCX/RCA) differences in coronary flow patterns, and their implications on ischemic diseases.
CORONARY TERRITORIES SHOWN ALONGSIDE THE VASCULAR NETWORK THAT SUPPLIES THE FLOW